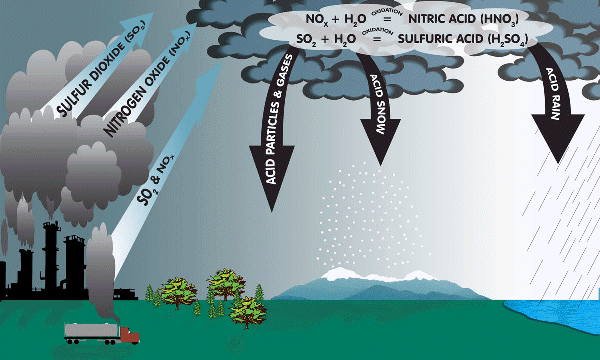
Acid Rain Formation
Lakes and rivers have slowly become more acidic in the last 20 years worldwide. Fishes have started to disappear since that time. Scientists know that most living plants and animals can only tolerate very small changes in the acidity. The gradual acidification of lakes and rivers around the world may be producing long-term harmful effects on fish and other aquatic organisms.
Industrial air pollution, such as burnt coals from electic power plants and automobile exhausts, release many gases into atmosphere. Some of these gases are nitrogen monoxide (NO), nitrogen dioxide (NO2), and sulfur dioxide (SO2). These gases can be transformed into acids when they enter the atmosphere. The acids formed are washed to the ground when rain falls. Acidic rainwater that are collected in bodies of water increase the water's acidity level.
Source: Phoenix Science Series - Chemistry by Estrella Elona Mendoza
No comments:
Post a Comment